&(%)D!(+) ,-"('
D!&,.+&'-,
" .+ -<9(F=;=BC:%=EI=8#IB7H=CB)CH9BH=5@G
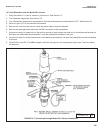
The figure shows a thin section through a pore in the junction plug. The junction separates a solution of potassium chloride on
the left from a solution of hydrochloric acid on the right. The solutions have equal molar concentration. Driven by concentration
differences, hydrogen ions and potassium ions diffuse in the directions shown. The length of each arrow indicates relative rates.
Because hydrogen ions move faster than potassium ions, positive charge builds up on the left side of the section and negative
charge builds up on the right side. The ever-increasing positive charge repels hydrogen and potassium ions. The ever-increas-
ing negative charge attracts the ions. Therefore, the migration rate of hydrogen decreases, and the migration rate of potassi-
um increases. Eventually the rates become equal. Because the chloride concentrations are the same, chloride does not influ-
ence the charge separation or the liquid junction potential.
%"*."#.'-"(')(-'-"%
2C@N<GO=MD?B@N@@$DBPM@DN<IDIO@BM<GK<MOJAOC@M@A
@M@I>@ @G@>OMJ?@ 'O KMJQD?@N OC@ @G@>OMD><G >JII@>ODJI
=
@OR@@IOC@M@A@M@I>@@G@>OMJ?@<I?OC@GDLPD?=@DIBH@<N
PM@?1<GO=MD?B@NO<F@<Q<MD@OTJAAJMHN<ITOCDIBAMJH<
BG<NNAMDOOJ<RJJ?@IKGPB1<GO=MD?B@N<M@CDBCGTKJMJPN
<I?OC@KJM@N<M@ADGG@?RDOCDJIN2C@DJIN>JH@AMJHOC@ADGG
DIBNJGPODJI<I?OC@N<HKG@1JH@=MD?B@NK@MHDOJIGT?DAAP
NDJIJADJINOCMJPBCOC@EPI>ODJI'IJOC@M?@NDBIN<NGJR
JPOAGJR JA ADGGDIB NJGPODJI J>>PMN +DBM<ODJI JA DJIN DI OC@
=MD?B@B@I@M<O@N<QJGO<B@><GG@?OC@GDLPD?EPI>ODJIKJO@I
OD<G2C@GDLPD?EPI>ODJIKJO@IOD<GDNDIN@MD@NRDOCOC@H@<NPM
DIB<I?M@A@M@I>@@G@>OMJ?@KJO@IOD<GN<I?DNK<MOJAOC@JQ@M
<GG>@GGQJGO<B@
$DBPM@ C@GKN DGGPNOM<O@ CJR GDLPD? EPI>ODJI KJO@IOD<GN
JMDBDI<O@2C@ADBPM@NCJRN<N@>ODJIOCMJPBC<KJM@DIOC@
N<GO =MD?B@ $JM NDHKGD>DOT <NNPH@ OC@ =MD?B@ >JII@>ON <
NJGPODJIJAKJO<NNDPH>CGJMD?@<I?CT?MJ>CGJMD><>D?JA@LP<G
HJG<M>JI>@IOM<ODJI'JINAMJHOC@ADGGDIBNJGPODJI<I?DJIN
AMJHOC@N<HKG@?DAAPN@OCMJPBCOC@KJM@N"DAAPNDJIDN?MDQ
@I =T >JI>@IOM<ODJI ?DAA@M@I>@N #<>C DJI HDBM<O@N AMJH
R
C@M@DON>JI>@IOM<ODJIDNCDBCOJRC@M@DON>JI>@IOM<ODJIDN
GJR @><PN@DJINHJQ@<O?DAA@M@IOM<O@N<>C<MB@N@K<M<
ODJI?@Q@GJKNNOC@>C<MB@N@K<M<ODJIDI>M@<N@N@G@>OMJ
NO<OD>AJM>@N><PN@OC@A<NO@MHJQDIBDJINOJNGJR?JRI<I?
OC@NGJR@MHJQDIBDJINOJNK@@?PK#Q@IOP<GGTOC@HDBM<
ODJIM<O@N=@>JH@@LP<G<I?OC@NTNO@HM@<>C@N@LPDGD=MD
PH2C@<HJPIOJA>C<MB@N@K<M<ODJI<O@LPDGD=MDPH?@O@M
HDI@NOC@GDLPD?EPI>ODJIKJO@IOD<G
*DLPD? EPI>ODJI KJO@IOD<GN @SDNO RC@I@Q@M ?DNNDHDG<M @G@>
OMJGTO@NJGPODJIN>JH@DIOJ>JIO<>O2C@H<BIDOP?@JAOC@
KJO@IOD<G?@K@I?NJIOC@?DAA@M@I>@=@OR@@IOC@HJ=DGDOTJA
OC@DJINGOCJPBCGDLPD?EPI>ODJIKJO@IOD<GN><IIJO=@@GDH
DI<O@?OC@T><I=@H<?@NH<GG<I?M@G<ODQ@GT>JINO<IO
NH<GGGDLPD?EPI>ODJIKJO@IOD<G@SDNONRC@IOC@DJINKM@N@IO
DI BM@<O@NO >JI>@IOM<ODJI C<Q@ @LP<G JM <GHJNO @LP<G
HJ=DGDOD@N2C@>PNOJH<MTR<TJAM@?P>DIBEPI>ODJIKJO@I
OD<GN DN OJ ADGG OC@ M@A@M@I>@ @G@>OMJ?@ RDOC >JI>@IOM<O@?
KJO<NNDPH >CGJMD?@ NJGPODJI 2C@ CDBC >JI>@IOM<ODJI
@INPM@NOC<OKJO<NNDPH>CGJMD?@DNOC@H<EJM>JIOMD=POJMOJ
OC@ EPI>ODJI KJO@IOD<G <I? OC@ I@<MGT @LP<G HJ=DGDOD@N JA
KJO<NNDPH<I?>CGJMD?@DJINH<F@NOC@KJO@IOD<GNH<GG
('/+-"' /(%- -(D!
#LP<ODJI NPHH<MDU@N OC@ M@G<ODJINCDK =@OR@@I H@<N
PM@?>@GGQJGO<B@DIH4K&<I?O@HK@M<OPM@DI)@GQDI
#2#
Y
22K&
2C@ >@GG QJGO<B@ #2aOC@ IJO<ODJI @HKC<NDU@N OC@
?@K@I?@I>@JA>@GGQJGO<B@JIO@HK@M<OPM@aDNOC@NPHJA
ADQ@@G@>OMD><GKJO@IOD<GN$JPM<M@DI?@K@I?@IOJAOC@K&JA
OC@O@NONJGPODJI<I?<M@>JH=DI@?DIOC@ADMNOO@MH#
Y
2
2C@N@KJO@IOD<GN<M@GDNO@?=@GJR
OC@KJO@IOD<GJAOC@M@A@M@I>@@G@>OMJ?@DIND?@OC@BG<NN
@G@>OMJ?@
OC@KJO@IOD<G<OOC@DIND?@NPMA<>@JAOC@BG<NNH@H=M<I@
OC@KJO@IOD<GJAOC@@SO@MI<GM@A@M@I>@@G@>OMJ?@
OC@GDLPD?EPI>ODJIKJO@IOD<G
" .+ +9:9F9B79@97HFC89
The fixed concentration of chloride inside the electrode
keeps the potential constant. A porous plug salt bridge at
the bottom of the electrode permits electrical contact
between the reference electrode and the test solution.